“It took scientists 2200 years, from Greece in 400 BCE to Europe in 1800, to grasp what elements really are, because most are too changeable,” says Sam Kean in his tantalizing book The Disappearing Spoon.
Categorizing and looking for patterns in matter started in ancient times. In Aristotle's time, water, fire, earth and air were considered the basis for everything. Over time, many observations and experiments, in addition to the discovery of new elements, led to the scientific classification system of elements based on common characteristics and behaviors known as the periodic table.
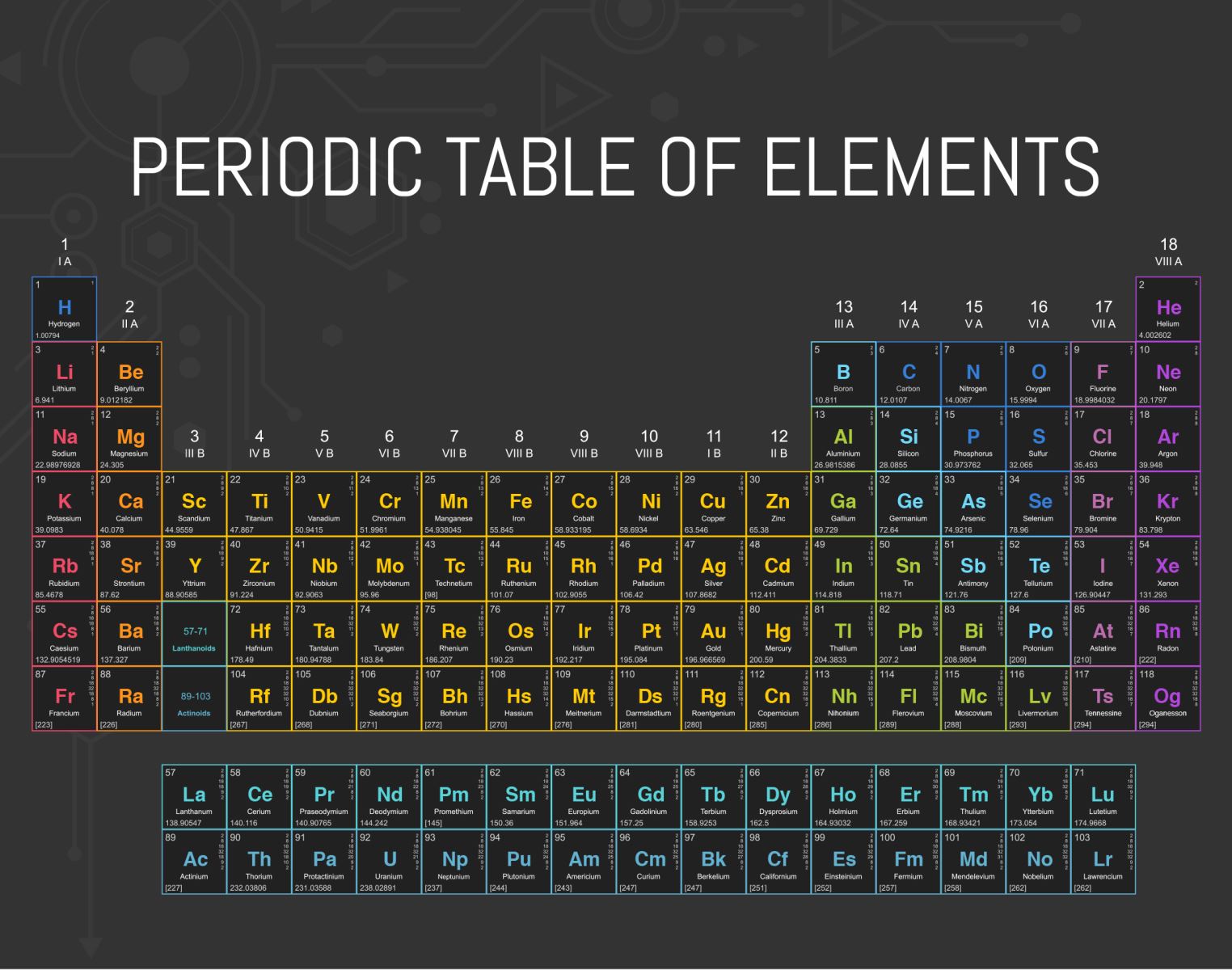
At the time Mendeleev arranged the elements in his periodic table, there were 63 elements known. The first element in the periodic table is hydrogen for it is the simplest element with a single electron orbiting one proton and no neutrons. Hydrogen can bond with other elements to form compounds; the most prominent example is when two hydrogen atoms bond with one oxygen atom to form H2O, or water. On the other hand, the last element to be added to the periodic table was ununoctium, also known as eka-radon or element 18; it has the temporary element symbol Uuo. Ununoctium has the highest atomic number and highest atomic mass of all discovered elements.
Out of the 118 elements on the periodic table now, only 92 elements are found naturally, some of which can only be created from other natural elements by radioactive decay(1). The remaining elements are synthetic elements produced with the help of humans, mostly in particle accelerators. The first element to be produced synthetically was technetium with the atomic number 43; every form of it is radioactive. Many of technetium’s properties were predicted by Mendeleev before the element was discovered.

One of the rarest elements in the world, francium was the last element discovered in nature and not by synthesis. It results from the breakdown and decay of actinium, and has an extremely short half-life; a matter of minutes. Outside the laboratory, francium is extremely rare; scientists estimate there are no more than a few ounces of it in existence on Earth at any given time. Francium has been used for research purposes in the fields of biology and atomic structure.
By mass, oxygen is the third most abundant element in the universe after hydrogen and helium, and the most abundant in the Earth’s crust making up almost half of the crust’s mass. It is a highly reactive non-metallic element that forms compounds with almost all other elements. Free oxygen is too chemically reactive to appear on Earth without the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water. Because it comprises most of water’s mass, oxygen comprises most of the mass of living organisms.
There are a total of 38 different types of transition metals between groups 3 and 12 on the periodic table. These metals are ductile, malleable and are good conductors of heat and electricity. Some of the most abundant transition metals include copper, cobalt, iron, and nickel; other less abundant types include scandium and niobium. Iron, cobalt and nickel are the only transition metals that can produce a magnetic field.
On the other hand, there are seven elements in the periodic table that have the properties of both metals and non-metals. These elements are classified as metalloids; they are boron, silicon, germanium, arsenic, antimony, tellurium, and polonium. Some metalloids, such as silicon and germanium, are semi-conductors, which makes metalloids useful in computers and calculators.
Noble gases, also known as inert gases, are a group of chemical elements with very similar properties under standard conditions; they are all odorless, colorless, with very low chemical reactivity. The six noble gases are helium, neon, argon, krypton, xenon, and the radioactive radon. The discovery of the noble gases aided in the development of a general understanding of atomic structure. Noble gases have several important applications in industries such as lighting, welding, and space exploration.
‘Noble’ indeed, helium, or element two, has exactly the number of electrons it needs to fill its only shell. This ‘closed’ configuration gives it tremendous independence because it does not need to interact with other atoms to share or steal electrons to be satisfied. The same configuration extends down the 18th column. Scientists might have figured out what elements are much sooner had they known about helium, which has never reacted with another substance and has never been anything but a pure element*.
One column to the west sits the most energetic and reactive gases on the periodic table, the halogens*. These are five non-metallic elements found in group 17 of the periodic table. The term "halogen" means "salt-former" and compounds containing halogens are called "salts". Halogens exist, at room temperature, in all three states of matter: solid (iodine and astatine), liquid (bromine), and gas (fluorine and chlorine).
If the table is a map, then to the east of the noble gases sits column one; even more violent elements known as alkali metals. Despite being normal metals in some ways, instead of rusting or corroding, alkalis can spontaneously combust in air or water. They also form an alliance of interests with halogen gases*.
The periodic table with its thrilling history and stunning phenomena is truly an eternally fertile ground for scientific discovery, so let us continue gazing through the magnifying glass and reflecting on what we see.
If you are interested in this topic, you will also like this interactive guide posted on the Periodic Table of Technology that shows the everyday use of elements in technology; created on The Beacon on Fios.Verizon.com, a Verizon authorized retailer.
 Glossary
Glossary
(*): quoted from The Disappearing Spoon by Sam Kean
Radioactive Decay is the spontaneous breakdown of an atomic nucleus resulting in the release of energy and matter from the nucleus. There are three types of radioactive decay are called Alpha Decay, Beta Decay and Gamma Decay. You can learn more by visiting:
http://www.ndt-ed.org/EducationResources/HighSchool/Radiography/radioactivedecay.htm
References
chemistry.about.com
www.ehow.com
www.chemicalelements.com
www.ndt-ed.org
en.wikipedia.org
The original article was published in the PSC Newsletter, Summer 2011 issue.