 |
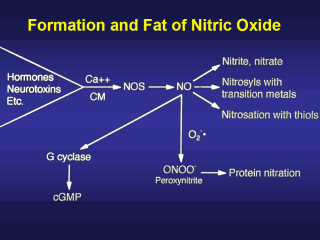
One of the most
rapidly advancing areas of clinical application is the inhalation of low
concentrations of the NO gas. Interestingly, nitric oxide at low
concentrations is quite stable and minimally reactive. Its reactivity and
toxicity is a second order reaction dependent upon the concentration of
nitric oxide and its interaction with other free radicals and reactive
oxygen species such as superoxide. At higher concentrations NO can interact
with many transition metals, heme-containing proteins, thiol groups and can
oxidize functionalities on polynucleotides (RNA and DNA) and proteins and
can form strand breaks in polynucleotides. There is a rapid and almost
diffusion-limited interaction of nitric oxide with superoxide to form the
very reactive peroxynitrite.
|
