 |
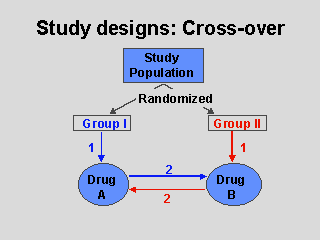
In a planned cross-over design, all participants receive both study
treatments (e.g., both Drug A and Drug B, or Drug A and placebo); after a
trial period with the first therapy the patients are "crossed over" to the
other therapy. Thus participants are given two or more treatments in
sequence. As shown in this slide, participants are randomized to either
Group I or Group II, which determines the order of the therapies - in this
case, Group I will receive Drug A first, followed by Drug B, and Group II
will receive Drug B first, followed by Drug A.
Because each patient serves as his/her own control, this design is more
efficient than a non-crossover, parallel group type of design, thus allowing
smaller sample size. However, because of the possibility that carry-over
effects from the first to second period may be present (where effects of the
first treatment may still be present for a long period, or where the first
treatment permanently changes the course of the disease), cross-over trials
cannot be used for certain types of treatment comparisons. Methods to
minimize carry over effects and to test for the presence of carry-over are
available.
|
