| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |review |
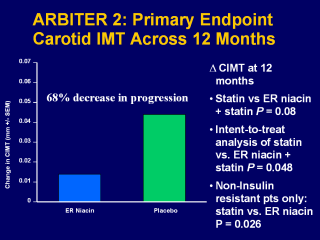 |
The change in CIMT from baseline to 12 months was 0.044 mm in the placebo group (P<0.001) and 0.014 mm in the ER-niacin group (P=0.023). Thus, the increase in CIMT was 3-fold greater in the placebo than in the ER-niacin group. There was a trend towards significance for the between-group comparison of the rate of progression for the placebo and ER-niacin groups (P=0.08). Taylor AJ, et al. ARBITER 2: A double-blind, placebo-controlled study of extended-release niacin on Atherosclerosis progression in secondary prevention patients treated with statins.
|