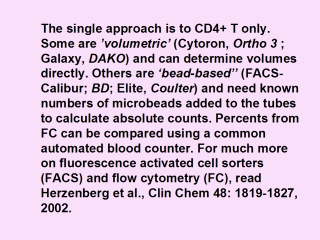
 |
In order to
introduce affordable CD4 tests into practice, 6 issues need to be
considered: 1/ Advantages of volumetric ‘single-platform’ cytometers in
terms of running costs, 2/ Introduction of primary CD4 gating 4 with the use
of a single CD4 reagent (instead of 6-9 reagents as currently used
expensively), 3/ The availability of monoclonal CD4 antibodies 4 conjugated
to fluorochromes detectable with inexpensive red diode lasers, 4/ Fixatives
for short term use, e. g., TransFix from NEQAS, UK, 5/ Standard cells 1
fixed for long periods to facilitate participation in international quality
control and 6/ A search for simple volumetric flow cytometers that handle
well in tropical conditions. See Janossy, Jani & Goehde Br J Haemat
111, 2000.
Enumeration of CD4 T lymphocytes is the most sensitive
indicator for assessment. This indicator, together with the estimation of
plasma viral load constitutes 2 universally accepted monitoring tools aside
from antibody tests, reverse transcriptase (RT-PCR) and the Western blot
test separating viral proteins in gel. Tests based on the polymerase chain
reaction (PCR) are usually for DNA and here the reaction is reversed
just as retro- means reversed. EIA was given in the anterior
Diagnosis. EIA deserves more development as a cheap method for CD4+
counting.
|
