Guillain–Barré Syndrome (GBS) is a debilitating postinfectious autoimmune disorder that impairs the Peripheral Nervous System (PNS) neurons; GBS is the lead cause of acute neuromuscular weakness and paralysis worldwide. Symptoms cover a wide spectrum, both motor and sensory. In this article, we will explore GBS pathogenesis and treatment, and then conclude with a recent method that offers new hope against this disorder.
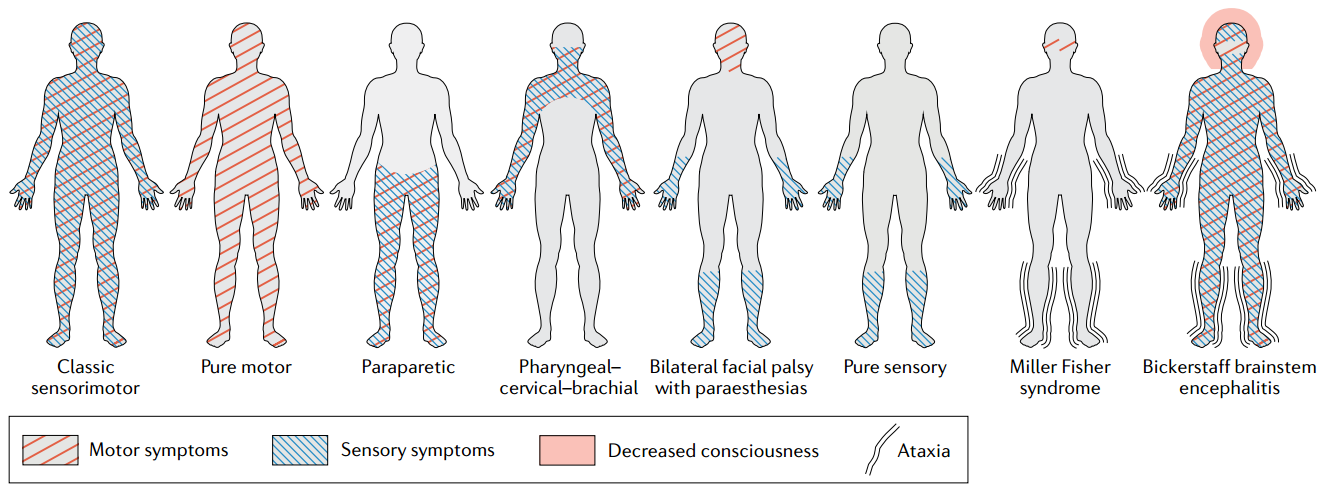
Pattern of symptoms in variants of Guillain–Barré syndrome. Credit: Nature.
Pathogenesis
The definitive GBS mechanism is unknown; however, the formation of cross-reactive autoantibodies against neuron gangliosides is a critical step in pathogenesis. GBS can occur due to bacterial or viral infections; bacteria include Campylobacter jejuni (C. jejuni), Haemophilus influenzae, and Mycoplasma pneumonia, while viruses include Cytomegalovirus, Varicella-Zoster virus, and Epstein-Barr virus. Being a common GBS pathogen, we shall use C. jejuni as a model to explain this step.
Immunoglobulins (Igs) or antibodies are glycoproteins (proteins with sugar molecules) secreted in response to antigens (immune system stimulants). C. jejuni have antigen molecules known as Lipo-oligosaccharides (LOS). Like all other antigens, LOS are presented by Antigen-Presenting Cells (APCs) to T-cells, and B-cells for recognition. B-cells become plasma cells that secrete antibodies against LOS. The problem is the molecular mimicry between gangliosides and LOS, which could turn LOS antibodies against gangliosides, leading to impairing neurons.
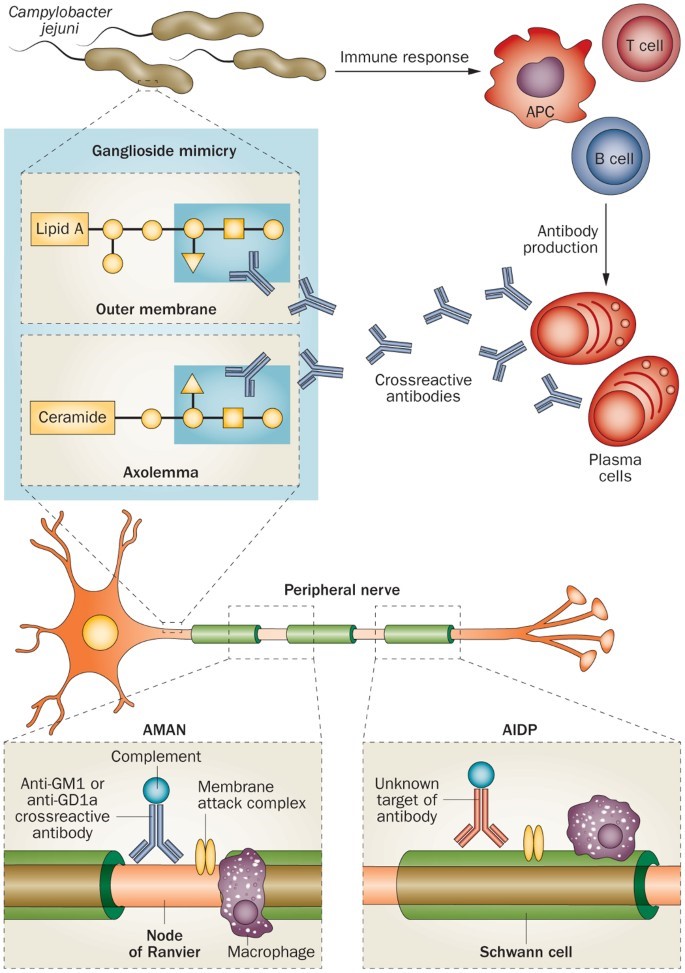
Molecular mimicry and antiganglioside antibodies. Credits: Nature.
Treatment
The past three decades witnessed great advancement in understanding GBS, which, unfortunately, did not reflect in treatment. The only two established treatments are Plasma Exchange (PE) and Intravenous Immunoglobulins (IVIgs); both are of equal efficacy and work best in early interventions. Like the pathogenesis process, the definitive mechanisms of treatment are not yet established.
In PE, the patient’s blood is separated into its liquid part, the plasma, and cells, then replacing plasma with another fluid. It is hypothesized to remove harmful molecules, such as autoantibodies from patients. Although PE became the gold standard in American Academy of Neurology (AAN) guidelines, it is largely inaccessible and costly.
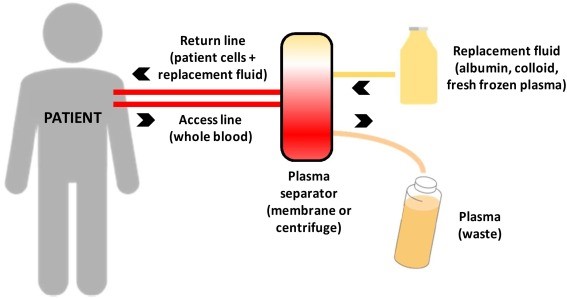
Therapeutic plasma exchange. Credits: ScienceDirect.
IVIgs consist of purified concentrated immunoglobulins taken from the blood of thousands of healthy donors. Hypothesized mechanisms include immunomodulation through suppressing and neutralizing autoantibodies.
Modified Zipper Method
Although the Zipper Method is a new method for treatment, the Zipper does not involve new therapeutic agents, but only uses the PE and IVIg in a new way. The procedure entails PE sessions after GBS diagnosis, followed by IVIg infusion, and so on for five cycles. Every two cycles are separated by a 24-hour interval. The Zipper Method has succeeded in reducing mortality and hospital stay.
In October 2021, a modification to the Zipper Method that sought easier applicability was published. Modifications included longer intervals between cycles (48 hours) and more cycles (7–10). Trials on four children with severe GBS and other autoimmune neurological disorders showed zero mortality and excellent outcome after follow-up for six months to two years. May this method become a new hope for all GBS patients.
References
biomedres.us
journals.sagepub.com
nature.com
nature.com
tandfonline.com
thieme-connect.com
Banner Image: https://www.foxnews.com/health/guillain-barre-syndrome-what-is-it